Authors:
Elisabetta Raganella Pelliccioni, Riccardo Nardelli, Lorenzo Serra, Fernando Spina
ISPRA – Isituto Superiore per la Protezione e la Ricerca Ambientale
March 2018
Recommended citation:
Raganella-Pelliccioni E., Nardelli R., Serra L, Spina F., 2018. The reintroduction of Bonelli’s Eagle (Aquila fasciata Vieillot 1822) in Sardinia: feasibility plan. Action A1, LIFE PROJECT “AQUILA a-LIFE”, March 2018.
1. Summary
INTRODUCTION
1 BONELLI’S EAGLE LIFE HISTORY
1.1 Status and distribution
1.2 Food habits
1.3 Competition
1.4 Demography
1.5 Dispersal and movements
2 THE PROJECT SITE
2.1 Description
2.2 Bonelli’s eagle in Italy and Sardinia: historic and present distribution
2.3 Analysis of the extinction causes
3 FEASIBILITY PLAN
3.1 Objectives
3.2 Evaluation of electrocution and illegal killing risks
3.3 Measures to minimize the electrocution and illegal killing risk
3.4 Prey availability
3.4.1 Analysis of population control data and hunting bags
3.5 Golden eagle distribution
3.6 Compliance with IUCN criteria for reintroduction
4 OPERATIONAL PLAN
INTRODUCTION
Bonelli’s eagle (Aquila fasciata Vieillot, 1822) is an amazing predator that disappeared in silence during the last few decades in Sardinia, most likely due to illegal taking for the black market. However, the island maintained all the characteristics to host the species in the long term. To promote the return of the Bonelli’s Eagle in Sardinia a joint effort with GREFA, Diputación Foral de Álava – Arabako Foru Aldundia, Fundació Natura Parc, Gestión Ambiental de Navarra S.A. and Ligue pour la Protection des Oiseaux resulted in the life project “Accomplish Western Mediterranean Bonelli's Eagle recovery by working together for an electricity grid suitable for birds”, AQUILA a-LIFE, awarded by the European Commission in 2017 and supported by the Ministry of the Environment and by Sardinian Regional Administration. The project aims at increasing the distribution of Bonelli's eagle in the western Mediterranean, reversing the negative trend shown by the species: the reintroduction of the species in Sardinia, under the responsibility of Ispra as associated beneficiary, is an important step of this process.
The present document represents the feasibility plan for the reintroduction, as foreseen by the project under the Action A1 “Estudio de viabilidad de la reintroducción en Cerdeña”.
1 BONELLI’S EAGLE LIFE HISTORY
1.1 Status and distribution
The Bonelli’s Eagle (Aquila fasciata Vieillot, 1822) is a medium to great size raptor (length: 55–65 cm in, wingspan: 150 cm) living in hilly or mountainous habitats, with rocky walls or crags and open to wooded land, in arid to semi-moist climate, from sea level to 1500 m. Temperature is a limiting factor for distribution, so that areas with coldest month isotherm below 2° are avoided (Parellada et al., 1984). Its wide geographical distribution includes the Mediterranean coast from the Iberian peninsula and Maghreb to the Middle east, the Arabic Peninsula and the southern Asia from Iran to eastern China. The Sunda islands in Indonesia host the subspecies renschi. The species is usually resident. The world population ranges from 20.000 to 49.999 individuals (BirdLife International, 2018), the most of which live in the Indian subcontinent and in southern China.
The largest part of the Paleartic population (2.000-3.000 breeding pair) is located in the Iberian peninsula (800-900 pairs) and Maghreb (Morocco, Algeria, Tunisia, 700-1.300 pairs) (Ponchon 2011).
The European population of Bonelli’s eagle was estimated in 880-1000 breeding pairs in ’90 of the past century (Tucker & Heath, 1994) after having faced a strong decrease during the previous 2-3 decades, related in large part to pollution caused by organochlorin pesticides. In Spain a minimum of 15-20 % of the breeding population was thought to have disappeared from 1980 to 1990 (Arroyo et al. in Tucker & Heath, 1994). Serious declines were recorded in southern France from 80 to about 30 pairs during the period 1960-1990 (Ponchon 2011), Cyprus and Sardinia (from about 30 to 0 pairs from ‘70s to ‘90s, see below). During 1990-2000 the species continued to be classified as Endangered (IUCN criteria) due to a marked decline of the Spanish population (-20-29%, BirdLife International 2004), although 658-721 pairs estimated in 2000 (Real, 2003) and some populations apparently stable.
Data referred to the period 2001-2012 show that the trend of the European breeding population is relatively stable, with local increases (namely French and Italian population) (BirdLife International, 2017). The current European population consists of 1100-1200 pairs. The conservation status was categorized by as Near Threatened in Europe (BirdLife International, 2017), and Least Concern at global scale (BirdLife International, 2018).
Across the Mediterranean range, the Bonelli’s Eagle occurs in a very wide range of habitats: desert-steppes, low maquis (e.g. “phrygana”), shrublands, evergreen woodlands or groves (Quercus ilex, Q. suber, Q. rotundifolia, , Pinus spp., Eucalyptus spp. plantations) or mosaics of wild vegetation and dry fields of low intensity farming (wheat, vineyards), sometimes wetlands (especially frequented by juveniles). In general mountainous habitats with available cliffs for nesting are preferred by adults, while non-adults disperse in hilly or lowlands habitats (Carrascal & Seoane, 2009).
Eyries are massive structure of sticks (up to 2 m of diameter) located on cliff ledges, less frequently on large-trees (tree nesting is usual in particular contest, e.g. Portugal (Palma et al., 2011). Nests are re-used in successive years, and 2-5 nests can be used alternatively in different breeding seasons.
1.2 Food habits
The main preys of the Bonelli’s Eagle are medium size mammals (in particular European rabbits Oryctolagus cuniculus and hares Lepus sp.) and birds, like partridges (Alectoris rufa), pigeons (Wood pigeon Columba palumbus, Feral pigeon C. livia domestica), crows (Corvidae: Jackdaw Corvus monedula, Hooded crow Corvus corone, Jay Garrulus glandarius, Magpie Pica pica), sometimes gulls and herons, rats, squirrels, less often reptils.
The raptor is quite adaptable in diet, being the spectrum of preys variable among different part of the range, according to local prey availability. Ontiveros (2016) reports the following diet composition: rabbits (28%), pigeons (24%), partridges (15,3%), other mammals (7%), crows (7%), reptils (6,4%). In Catalonia, a study on food pellets revealed a diet consisting of European rabbit (27%), Columbidae (32.5%) and Red partridges (11.7%) (Resano, 2011). In Greece the rabbit represent 40.8 % of rests in food pellets, whereas birds account for 32.4% (Kastritis 2011). In Cyprus, partridges account for 30-33% as proportion of preys in food pellets, rats 29-33% Columbidae 14-26%, Corvidae 2-7%, with food spectrum ranging according to breeding stage (Iezekiel et al., 2011). Changes in diet due to seasonal prey availability are also reported, showing birds prevailing (80%) from August to April, and mammals from May to July, as rabbits become more abundant (Cramp & Simmons 1980). The diet of juveniles in dispersal appears richer in rabbit than that of breeding adults (Moleón et al., 2009).
The preys are searched by patrolling and taken on ground after stoop from perches or soaring position; birds are often catched in flight with ambushes from cover of trees as they take-off. Frequently the raptor hunts in pair. The frequency of catching rabbits is more affected by prey accessibility (due to a lower vegetation coverage) than prey abundance (Ontiveros et al., 2005).
1.3 Competition
In the Mediterranean region, Bonelli’s eagles coexist simpatrically with Golden eagles (Aquila chrysaetos). Although the two eagles can nest quite closely without apparent hostility, interspecific competition is reported, sometimes by means of mutual exclusion from their respective territories (Thiollay 1968, Cheylan 1973 in Cramp & Simmons 1980). Competition for food and occupation of territories previously inhabited by Bonelli’s eagle (impeding recolonization) have been proposed as the main competition mechanisms (López-López, 2011). However the presence of neighbour Golden eagle breeding pairs is considered as negligible for productivity, being this parameter negatively affected only in areas with high density of Golden or Bonelli’s eagles, or in breeding pairs composed by pre-adult Bonelli’s eagles (Carrete et al. 2006).
During hatching, Bonelli’s eagle males frequently show aggressive behaviour and pursuits against intruding Golden eagles, as well as Griffon vultures or Booted eagles, although most other diurnal raptors are tolerated; likewise, Bonelli’s eagles intruders in golden eagles territories can be attacked, with possible fatal consequences.
Pairs are highly territorial and defend their territories from neighbour pairs by visual displays consisting of typical undulating flights, with sequences of aerial plunges and rises. Direct attacks to conspecific birds are mainly launched by males during the breeding period. Immatures are often tolerated by adults, even in August-September, possibly being young birds of the previous breeding season. Territories are variable in size reflecting differences in quality, the majority extending 50-130 km2, with normal activity radius of 5-6 km around the eyrie during the breeding period (Thiollay 1968 in Cramp & Simmons 1980). Recent studies on Bonelli’s eagles tagged with GPS devices revealed very little overlap between home-ranges of different neighbour pairs, and a higher quality in smaller home-ranges (Rosario et al. 2011).
Pair density is variable among different areas. In Spain, the average distance between neighbour pairs is 11.9 km, ranging between 8.2 km (highest density in the Penibétic Cordilleras) and 33.7 km (Ebro basin, Arroyo et al., 1995).
1.4 Demography
A long-term study on twelve populations, representing the largest European metapopulation (Iberian peninsula plus southern France populations) allowed to estimate adult survival and yearly productivity (Hernandez et al. 2011). According to a geographical gradient, southern populations showed the highest survival rate (> 0.91) while those populations located from Murcia to France showed a lower survival (<0.87). In the French population, pre-adult survival was estimated at 0.5 for fledgings, 0.57 for one- or two year-olds, and at 0.86 for three-year-olds and older individuals.
Even productivity varied among different areas: southern populations showed the highest productivity (>1.3 chicks per pair) while the western and northern populations showed a lower productivity (<0.8 chicks per pair). In agreement with a sink-source model, the southern populations allow the maintenance of the other populations. In addition, several factors contribute to variations in productivity: availability of suitable cliffs and south-oriented nests (Ontiveros & Pleguezuelos, 2003), low altitude and habitat heterogeneity (López-López et al., 2007) can improve the breeding performance, while prey reduction (Fernández et al. 1993), human disturbance and the presence of breeding pre-adult pairs (Balbontín et al., 2003) can reduce productivity.
Adult (Hernandez et al. 2011, Ravayrol 2011) and juvenile survival (Ravayrol 2011) are indicated as trigger demographic parameters on the population growth rate of the whole metapopulation.
Direct persecution and electrocution are the main unnatural mortality factors. Destruction of habitats, limited access to trophic resources, rural abandonment and anthropisation (urban expansion, infrastructures, wind farms and industrial photovoltaic installations), human disturbance and nest poaching are further potential threats for the Bonelli’s eagle populations.
1.5 Dispersal and movements
The species is resident throughout its range, although adults are less attached to their territories during post–breeding period. Juveniles abandon their native territory in August, 77-113 days after fledging (Real et al., 1998) and will disperse up to 200 km and individuals occasionally wander further afield and pass through key migration routes (Shirihai et al. 2000, Ferguson-Lees & Christie 2001). During dispersion, juvenile use to temporarly settle in areas with no territorial adult pairs and abundance in prey. The settlement takes places after juvenile become 245 days old on average (n=16); after 500 days males spend a longer time than females exploring surrounding areas (Balbontín & Ferrer, 2009).
2 THE PROJECT SITE
2.1 Description
 Sardinia is the second largest island (24.100 km²) in the Mediterranean, following Sicily. It is 270 km long (North to South) and 145 km wide (–East to West) and it is located 11 km South from Corsica (France). Sardinia is located 188 km apart from the Italian peninsula and 178 km apart from the African continent.
Sardinia is the second largest island (24.100 km²) in the Mediterranean, following Sicily. It is 270 km long (North to South) and 145 km wide (–East to West) and it is located 11 km South from Corsica (France). Sardinia is located 188 km apart from the Italian peninsula and 178 km apart from the African continent.
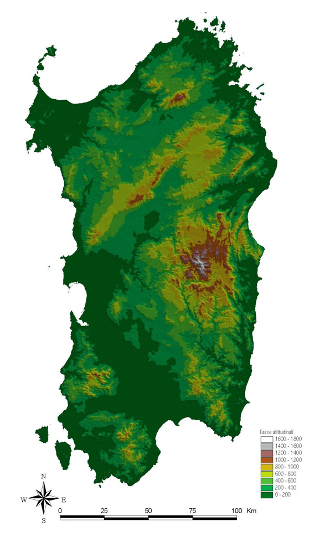
The island is characterized by a variety of habitats and landscapes (Fig. 1), like short plateaus, small massifs, more or less isolated, and hills with modest elevations; these reliefs are the result of the erosion of an ancient crystalline massif made up mainly by granite and other rocks, corrugated and already emerged in the Palaeozoic. 14% of the whole surface is covered by mountain, up to 1834 m asl (Gennargentu massif); hills and rocky plains make up 68% of the territory. There are also several isolated massif like Limbara mountain (SS), Sette Fratelli and Sulcis (CA). Main river basins are Tirso, Coghinas, Flumendosa, Temo (the largest of the region, 151 km) and Cedrino. There is only one natural lake, Baraz, but artificial basins are quite common. The island is covered by Mediterranean vegetation, with Quercus ilex and the less common Quercus suber.
The maquis can be very dense and it is populated by Pistacia lentiscus, Arbutus unedo, Erica arborea, Cistus, Genista, Calicotome, Myrtus communis, Rosmarinus officinalis, Lavandola stoechas and Euforbia dendroides.
The gariga is also well represented as an alteration of the maquis, with dominance of Helichrysum italicum, Thymus herba-barona. Sardinia hosts a wide variety of animal species, many of them endemic as a subspecies. Mammals include red fox Vulpes vulpes, wild cat Felis silvestris lybica, weasel Mustela nivalis boccamela, martens Martes martes, all possible predators of the chicks during the hacking phase. Other mammalian species of interest as possible prey are the wild rabbit Oryctolagus cuniculus huxleyi, Sardinian hare Lepus capensis, Rattus sp., hedgeog Erinaceus europaeus. As for birds, partridge Alectoris barbara is quite common in the island; as possible competitor, Golden eagle Aquila chrysaetos is widespread in the region.
2.2 Bonelli’s eagle in Italy and Sardinia: historic and present distribution
Once widespread and relatively abundant in Sicily, Sardinia and in the southern regions of Italy, the species was considered rare and irregular in central and northern Italy (Moltoni 1945). It is currently breeding only in Sicily, while there are no valid reports of breeding attempts or sightings for Sardinia since few decades. In Sicily, 44 territorial pairs have been reported in 2016, with 28 fledged young (2016, LIFE14 NAT/IT/1017 – ConRaSi). It is reported as rare and irregular in Calabria, with no confirmed territorial pairs.
The sharp decline of the species in Italy has been due to direct persecution, which is still a well documented threat in Sicily, mainly for collection purposes and falconry. Illegal trade of eggs and chicks taken from Sicilian pairs has been reported several times in recent years. Other causes are the decline of prey species, direct persecution as pest species until its full protection (1977), use of biocides in agriculture (Massa 1976). The species is presently reported as critically endangered in the “Red List of Italian Vertebrates” (Rondinini et al. 2013).
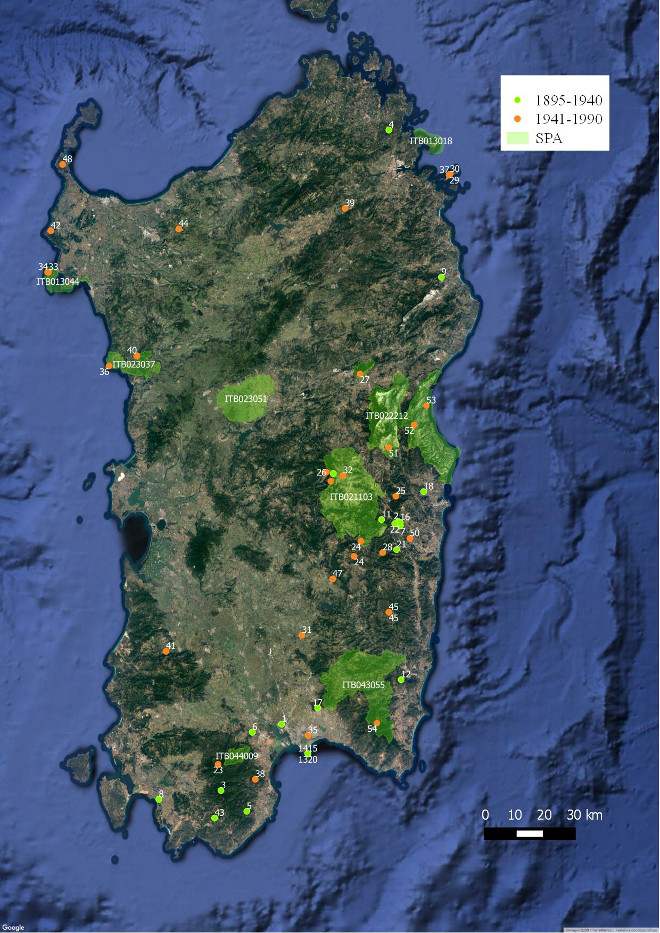
In Sardinia, Bonelli’s eagle was once widespread and the species was considered very common (Cara 1842) (Fig.2 and table 1). In the first half of the 1900 the species is reported in the south-eastern part of the island. Later, sightings cover approximately the whole island, even if an increase of interests in the species might have improved the knowledge of its distribution. In 1972, Schenk reported a population of 30 pairs for the island. The last confirmed observation is dated 1990, even if it is possible that few remaining individuals were still present in the island at that time. Despite the increasing interests in both birdwatching and naturalistic photography, the extinction of species in Sardinia occurred in the general indifference and the territories once inhabited by the species were probably occupied by the Golden Eagle.
2.3 Analysis of the extinction causes
Between early seventeenth and the middle of the twentieth century the persecution of raptors resulted in a sharp decline of the species and national extinctions across Europe. Later on, thanks to an increasing environmental awareness coupled with a growing influence of conservationist association, the fate of many birds of prey start to change. Unfortunately, the fortune of the Bonelli’s eagle in Sardinia followed the same path, although the species did not recover and was driven to extinction. Extinction causes are not well known: according to Schenk (1976) direct persecution by shepherds and hunters has been a key factor leading to the decline of the species, along with the removal of chicks and eggs for collection. Nowadays the traditional perception of raptors as a pest species has changed also thanks to an increasing public and political interest in species and habitat conservation and to the enacting of protection laws. A change in attitude has occurred also in Sardinia as demonstrated for example by the lack of persecution against the Golden Eagle (Aquila chrysaetos), whose population has shown a significant recovery from previous periods and has considerably increased in the last decades.
|
N |
Site |
Province |
Date |
Year |
Collection/References |
|
1 |
Elmas |
Cagliari |
5/04/1896 |
1896 |
Coll. Arrigoni degli Oddi, Roma |
|
2 |
Lanusei |
Nuoro |
20/12/1897 |
1897 |
Coll. Arrigoni degli Oddi, Roma |
|
3 |
San Pantaleo |
Cagliari |
15/6/1898 |
1898 |
Coll. Arrigoni degli Oddi, Roma |
|
4 |
San Pantaleo |
Sassari |
15/6/1898 |
1898 |
Coll. Arrigoni degli Oddi, Roma |
|
5 |
Pula |
Cagliari |
10/09/00 |
1900 |
Coll. Arrigoni degli Oddi, Roma |
|
6 |
Assemini |
Cagliari |
08/10/00 |
1900 |
Coll. Arrigoni degli Oddi, Roma |
|
7 |
Lanusei |
Nuoro |
10/10/00 |
1900 |
Coll. Arrigoni degli Oddi, Roma |
|
8 |
Stagno di Villarios |
Cagliari |
feb-01 |
1901 |
Coll. Arrigoni degli Oddi, Roma |
|
9 |
Siniscola/Torpé |
Nuoro |
feb-01 |
1901 |
Coll. Arrigoni degli Oddi, Roma |
|
10 |
Gennargentu |
Nuoro |
31/08/06 |
1906 |
Coll. Arrigoni degli Oddi, Roma |
|
11 |
Lanusei |
Nuoro |
15/10/36 |
1936 |
Ragionieri - Coll. Foschi ,Forlì |
|
12 |
Muravera |
Cagliari |
25/05/39 |
1939 |
Ragionieri - Coll. Foschi, Forlì |
|
13 |
Cagliari |
Cagliari |
9/2/1895 |
1895 |
Natural History Museum, Milan |
|
14 |
Cagliari |
Cagliari |
14/01/02 |
1902 |
Moltoni E., 1971 |
|
15 |
Cagliaritano |
Cagliari |
gen-06 |
1906 |
Moltoni E., 1971 |
|
16 |
Lanusei |
Nuoro |
19/01/07 |
1907 |
Ispra Zoological Collection, Ozzano Emilia |
|
17 |
Monti di Sinnai |
Cagliari |
30/09/08 |
1908 |
Ispra Zoological Collection, Ozzano Emilia |
|
18 |
Lotzorai |
Nuoro |
07/05/15 |
1915 |
Moltoni E., 1971. Atti Soc. It. Sci. Nat., 1926, pag. 161, quoted by Moltoni |
|
19 |
Sardegna |
|
|
1921-26 |
Moltoni E. e Sciacchitano J., 1926 |
|
20 |
Cagliari |
Cagliari |
1930 |
1930 |
Zoological Museum, Cagliari University; Bollettino Soc. Sarda Sci. Nat.; Cara G., 1842 |
|
21 |
Jerzu |
Nuoro |
06/02/34 |
1934 |
Ispra Zoological Collection, Ozzano Emilia |
|
22 |
Lanusei |
Nuoro |
21/02/34 |
1934 |
Ispra Zoological Collection, Ozzano Emilia |
|
23 |
M. Is Caravius (Siliqua) |
Cagliari |
27/03/56 |
1956 |
Bezzel E., 1957 |
|
24 |
Seui Cantoniera (Arquerì) |
Nuoro |
24/06/57 |
1957 |
Moltoni E., 1971 |
|
25 |
Villagrande |
Nuoro |
27/06/57 |
1957 |
Moltoni E., 1971 |
|
26 |
Gennargentu, Desulo |
Nuoro |
19/04/62 |
1962 |
Kunkel P., 1963 |
|
27 |
M. Ortobene |
Nuoro |
20/04/62 |
1962 |
Kunkel P., 1963 |
|
28 |
S. Barbara (Ulassai) |
Nuoro |
10/04/65 |
1965 |
Sudhaus W., 1966 |
|
29 |
Tavolara (Passo Malo) |
Olbia Tempio |
27/02/66 |
1966 |
Moltoni E., 1971 |
|
30 |
Tavolara |
Olbia Tempio |
21/09/67 |
1967 |
Moltoni E., 1971 |
|
31 |
Senorbì |
Cagliari |
03/12/70 |
1970 |
Mocci Demartis A. & Restivo De Miranda M.A., 1978 |
|
32 |
Bruncu Spina (Gennargentu, Desulo) |
Nuoro |
29/09/70 |
1970 |
Toso S., 1972 |
|
33 |
Punta Cristallo |
Sassari |
nov-74 |
1974 |
Torre A., 1980 |
|
34 |
Punta Cristallo |
Sassari |
19/06/76 |
1976 |
Torre A., 1980 |
|
35 |
Molentargius |
Cagliari |
13/03/78 |
1978 |
Mocci Demartis A., 1980 |
|
36 |
Capo Marrargiu, Bosa |
Oristano |
Jul-83 |
1983 |
G. Sirigu, ined. |
|
37 |
Tavolara |
Olbia Tempio |
|
60ties |
G. Sirigu, ined. |
|
38 |
Monti di Capoterra (Conca d'Oru) |
Cagliari |
|
70ties |
G. Sirigu, ined. (reported sightings) |
|
39 |
Gallura |
Olbia Tempio |
|
70ties |
G. Sirigu, ined. |
|
40 |
Bosa-Montresta |
|
|
70-'80 |
G. Sirigu, ined. |
|
41 |
From Arbus to Portoscuso (south western Sardinia; Capo Pecora, Is arenas, Fluminimaggiore, Capo Altano, Monte Arcuentu, area Nebida-Masua) |
|
|
80-'90 |
G. Sirigu, ined. |
|
42 |
Nurra di Alghero (Argentiera) |
Sassari |
|
70-'80 |
G. Sirigu, ined. (reported sightings) |
|
43 |
Mountains of Teulada |
Cagliari |
|
before 1952 |
Steinbacher, 1952 |
|
44 |
Osilo |
Sassari |
|
before 1960 |
Martorelli, 1960 quoted by Schenk, 1976 |
|
45 |
Salto di Quirra (military area, Monte Cardiga, Murdega and Baccu Locci) |
Ogliastra |
|
before 1980 |
G. Sirigu, ined. |
|
46 |
Gennargentu, Girgini (Aritzo-Desulo), |
Nuoro |
|
before 1980 |
G. Sirigu, ined. |
|
47 |
Sarcidano (Orroli, Nurri, Lago del Flumendosa, Lago Mulargia) |
Cagliari |
|
before 1990 |
G. Sirigu, ined. |
|
48 |
Punta Falcone (Stintino? O Santa Teresa |
Sassari |
31/05/64 |
1964 |
Moltoni E., 1971 |
|
50 |
Ogliastra: Ierzu, Ulassai, Marina di Gairo (Capo Sferracavallo) |
Ogliastra |
|
before '80-'90 |
G. Sirigu, ined. |
|
51 |
Supramonte (Orgosolo, Urzulei) |
Nuoro-Ogliastra |
|
before '80-'90 |
G. Sirigu, ined. |
|
52 |
Baunei (Codule) |
Ogliastra |
|
before '80-'90 |
G. Sirigu, ined. |
|
53 |
Cala Luna |
Nuoro |
03/01/84 |
1984 |
Bogliani ined. |
|
54 |
Settefratelli |
Cagliari |
04/01/84 |
1984 |
Bogliani ined. |
Table 1. Observations of Bonelli’s eagle in Sardinia (Nissardi S., Zucca C. and Sirigu G., 2016, based on literature and unpublished observations).
3 FEASIBILITY PLAN
3.1 Objectives
The present plan aims to ascertain the persistence of ecological conditions suitable for the long term persistence of species in Sardinia, following its reintroduction.
Main characteristics accounted for are:
- Mortality risk by electrocution and illegal killing
- Presence and distribution of possible prey species
- Competition with Golden eagle
3.2 Evaluation of electrocution and illegal killing risks
To evaluate both the electrocution and illegal killing risks in the region, data from Recovery centres (CRAS) were analysed (tab.2).
Data are referred to injured birds and were obtained from CRAS (Wildlife recovery centres) of Bonassai (SS), Monastir (CA), Sulcis (CI, only illegal killing) and Ogliastra (OT). Data are not based upon systematic search and are not weighed by effort, therefore is likely they are locally biased. In any case they provide a picture of hot spots for electrocution and poaching, if any. As for illegal killing, only injuries caused by firearms are reported, therefore other form of illegal killing, like poisoning and trapping, are not considered here. However, these source of IK are less likely to affect Bonelli’s eagle, as also reported in the literature.
|
|
Provinces |
|
||||||||
|
|
CA |
CI |
MC |
NU |
OG |
OT |
SS |
SU |
tot |
|
|
Illegal Killing |
17 |
18 |
6 |
5 |
3 |
6 |
23 |
2 |
80 |
|
|
2014 |
3 |
11 |
|
2 |
|
3 |
5 |
|
24 |
|
|
2015 |
1 |
1 |
4 |
1 |
2 |
2 |
7 |
|
18 |
|
|
2016 |
9 |
6 |
1 |
2 |
1 |
|
3 |
|
23 |
|
|
2017 |
4 |
|
1 |
|
|
1 |
8 |
4 |
19 |
|
|
Electrocution |
|
|
|
5 |
3 |
5 |
17 |
|
30 |
|
|
2014 |
|
|
|
|
2 |
|
|
|
2 |
|
|
2015 |
|
|
|
1 |
|
2 |
6 |
|
9 |
|
|
2016 |
|
|
|
1 |
1 |
3 |
7 |
|
12 |
|
|
2017 |
|
|
|
3 |
|
|
4 |
|
7 |
|
Table 2. Illegal killing and electrocution casualties (N=110) in Sardinia provinces by years. CA: Cagliari, CI: Carbonia-Iglesias, NU: Nuoro, OG: Ogliastra; OT: Oristano; SS: Sassari; SU: Sud Sardinia. SU is operative since 2017 and it includes mainly the territories of CI and MC, plus other few municipalities once pertaining to other provinces.
Overall, illegal killing accounts for more than 70% of the causalities (4 years pooled), while the species Buteo buteo and Falcus tinnunculus sum up almost 50% of all the casualties.
|
Accipiter gentilis |
|
Corvus monedula |
|
Accipiter nisus |
|
Falco peregrinus |
|
Alauda arvensis |
|
Falco subbuteo |
|
Aquila chrysaetos |
|
Falco tinnunculus |
|
Ardea cinerea |
|
Gallinago gallinago |
|
Ardea purpurea |
|
Garrulus glandarius |
|
Athene noctua |
|
Recurvirostra avosetta |
|
Bubulcus ibis |
|
Scolopax rusticola |
|
Burhinus oedicnemus |
|
Streptopelia turtur |
|
Buteo buteo |
|
Sturnus vulgaris |
|
Circus aeruginosus |
|
Turdus philomelos |
|
Columba palumbus |
|
Tyto alba |
|
Corvus corone cornix |
|
Vanellus vanellus |
|
Corvus corax |
|
|
Fig. 3. Pooled casualties (N=110) of electrocution and illegal killing according to species. “Other species” are listed in the table. Mammals (Cervus elaphus corsicanus and Sus scrofa) are excluded.
According to the above data, illegal killing seems to be present in the region, but there is no area where these events can be considered recurrent and radicated (but see below). Similarly, apart from Falco tinnunculus and Buteo buteo, there is not any recurrent target species and Golden eagle, Aquila chrysaetos, only occurs once in the list of the species affected by illegal killing.
All these data support the hypothesis that illegal killing risk for Bonelli’s eagle in Sardigna can be considered as occasional and not an issue challenging the species reintroduction. Moreover, according to Andreotti et al. (2016) and Cauli F. (2009) in Sardinia illegal killing is mainly tailored on thrushes and it is practiced primarily between November and February in southern Sulcis and in the Sarrabus. Here, the catching techinques are traditionally represented by the hair of the horse, replaced today by nylon threads, arranged above a sprig among the vegetation so as to form a loop for the birds that perches on it. Nets and traps can also be used along with a berry –baited strap anchored to the ground by means of iron wire. Thrushes are killed for commercial purposes and can be sold to local caterers for the preparation of a typical dish, the "grive" (thrushes in Sardinian) with myrtle. Also in this case, since the catching techiniques are not selective, many other bird species are trapped in addition to the thrushes: among the most frequent victims, robins, Sardinian warbles, Sardinian partridge, chaffinches, hawfinches. Lastly, once very common in the island, the removal of eggs/chicks from nests of birds of prey for commercial purposes is currently not reported as an issue in Sardinia, while it remains a serious threat for several species, including Bonelli’s eagle, in Sicily.
3.3 Measures to minimize the electrocution and illegal killing risk
Main threat for the species is related to electrocution rather than to collision, it involves medium and low voltage power lines or distribution lines and it is due to birds making a connection between two live components. The electrocution risk is most commonly associated with poles and perching areas. In Sardinia, although many power lines are already retrofitted for electrocution risk, using for example the Elicord wires, there are still many pylon designs that represent a threat for the species.
 |
 |
| a. Suspended line: conductors are suspended to isolators | |
 |
 |
| b. Conductors lay on single or double isolators | |
 |
 |
| c. Boxer type with suspended or double isolator | |
 |
 |
| d. Pole disconnector (sx) and transforming box | |
Fig. 4. Main pole typologies existing in Sardinia (courtesy e-distribuzione).
According to data provided by the E-distribuzione company, in charge of the building and maintenance of the MV power lines, there are basically four types of poles in the region (Fig.4), all medium or highly risky for the species.
According to previous reintroductions, there are three main phases that can be identified when the species is particularly sensitive to the electrocution risk:
i) following the release, at the hacking site
Soon after the release from the hacking cage, the animals tend to remain in the nearby, increasing the distance from the release site as time passes, before disperse. At this stage, it is important to insulate all the poles that might be chosen as perch site by the animals. To minimize this risk, the presence of dangerous pylons will be checked within 1-5 km radius from the hacking site, well before the release, and the pylons will be retrofitted thanks to the commitment of E-distribuzione, who is willing to collaborate to reduce this risk at this stage;
ii) during dispersal phase
About 10 days after the release, eagles start to roam in the new area. Animals can cover up to 100 km from the release site. This is by far the most critical phase of the reintroduction because the risks for their survival cannot be properly evaluated and reduced. Although a constant monitoring is foreseen, by mean of GPS-UHF devices mounted on the released animals, it is particularly difficult to minimize of any possible risk for their survival, including electrocution risks, since the animals keep on changing site, although pylons in the most frequented areas by the juveniles should be retrofitted too.
iii) once established a territory
According to previous reintroductions carried out in Spain, quite often the eagles tend to established a territory where they were released, so it is highly probable that the animals will be back at the release site or its surroundings, after the dispersal phase. In any case, once established, it is possible to quickly identify pylons that can represent a risk and plan an adequate retrofitting. Although this activity is not foreseen within the project, ISPRA will look for additional funding instruments to cover the cost needed to retrofit the most dangerous power lines.
An agreement with E-distribuzione is going on to plan the most relevant actions to lower electrocution risk within the hacking site and to evaluate the costs for interventions on a wider area.
As for illegal killing risk, this is an unpredictable activity and can only be addressed through meetings tailored on main stakeholders, namely hunters and shepherds’ associations, but also general public. Indeed, the project foresees several meetings aimed also at evaluating the change of attitude in the key sectors. Previous experiences of the project partners in this field will help to define the contents of the meetings and the way the species reintroduction has to be presented. Among the communication activities, also schools within the release sites will be targeted by an education campaign, focused on the importance of the species as well as of the Natura2000 network.
3.4 Prey availability
Prey availability was indirectly assessed through hunting bags and population control data (corvids). All data were provided by Regional Administration. Although the use hunting statistics can provide a useful information as an index of population trends, they are potentially biased since not only dependent on abundance. Notably, hunting bags are dependent on hunting effort, linked to the selection of harvesting locations, the harvest strategy, the hunting seasons and hunters willingness to report; both- hunting effort and success - can be affected by weather conditions. Moreover, here data are available at province level resolution and considerable heterogeneity in species abundance and environmental conditions at this scale is highly likely. With data quality in mind, hunting bags descriptive statistics are reported to give an idea of the population trend of main potential prey species, in the last three years. Data reported are densities, computed on the area of hunting territory (i.e. excluding protected areas) for each province, as reported in the regional management plan (Apollonio et la. 2010). In order to shape the food choice of the released eagles, only most common prey species and those characterized by low hunting interest will be offered to eagles during hacking.
3.4.1 Analysis of population control data and hunting bags
Corvus corone cornix can be considered a common prey species of Bonelli’s eagle. In Sardinia the species is widespread, although at different density, also in smaller islands along the coast. It is present from the sea level up to Gennargentu Massif. Because of their impact on birds and on several hunted species as well as on agricultural production, the species is under population control in all the provinces of the region, but two (Carbonia-Iglesias and Nuoro), during the period when agricultural products are at risk of damage and during the reproductive period of birds species sensible to hooded crow predation (from march to – august). The species is also hunted regularly in all the provinces. Data related to hunting and population control are referred to different geographic areas within the region: while hunting is referred to territories when this activity can be exerted (thus outside protected areas), population control is carried out where needed, thus potentially everywhere. This makes it impossible to compute any density in relation to population control data. Moreover, while the hunting bag is unlimited, population control must not exceed 2000 individuals/province/year.
In 2015 (from 1/6 to 31/12) and 2016 (from 1/3 to 31/12) a total of 6998 individuals were removed (Fig. 5), using Larsen traps (28%), letter box (17%) and through shooting (53%; 1% unknown).
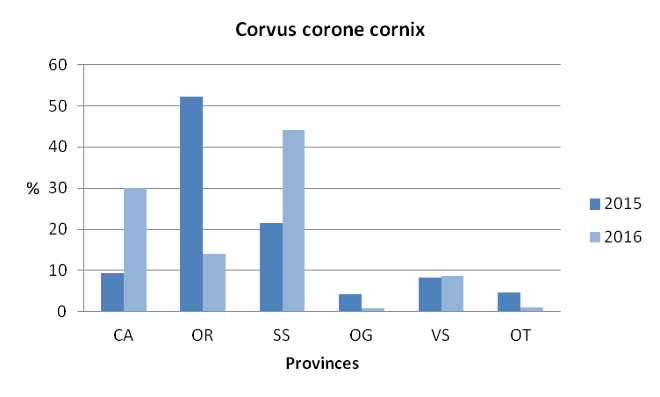
Data on population control of hooded crow, coupled with the known hunting bags, suggest that the species is quite common in the region and available as food source for the eagles in Sardinia.
Captured and shot crows might be used as a food resource during hacking phase and after, provided that the sanitary check does not highlight any relevant pathology dangerous for the eagle.
As for other potential prey species, although density of the concerned species changes in the three years depending upon the province considered (Fig. 6), values suggest an adequate availability of alternative preys at acceptable density. Although characterized by a wider food niche, in the last decades the golden eagle population and distribution increase in the region, suggesting that food availability should not be an issue for the species.
a.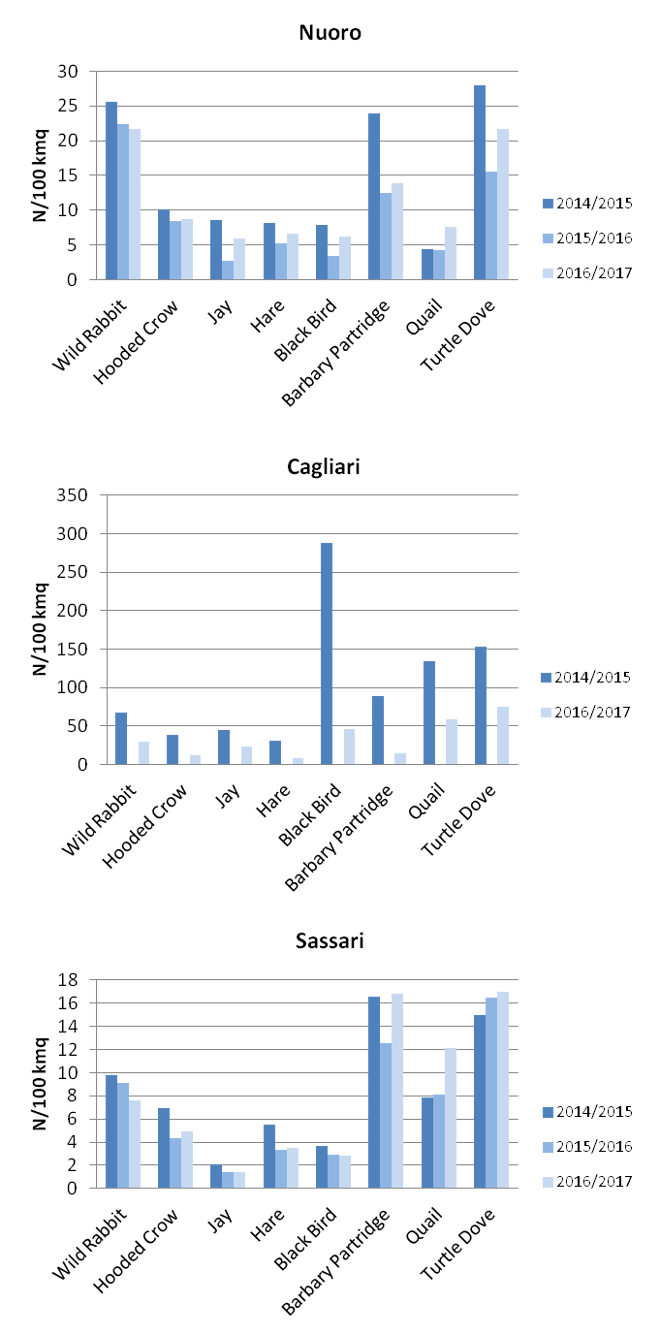
b.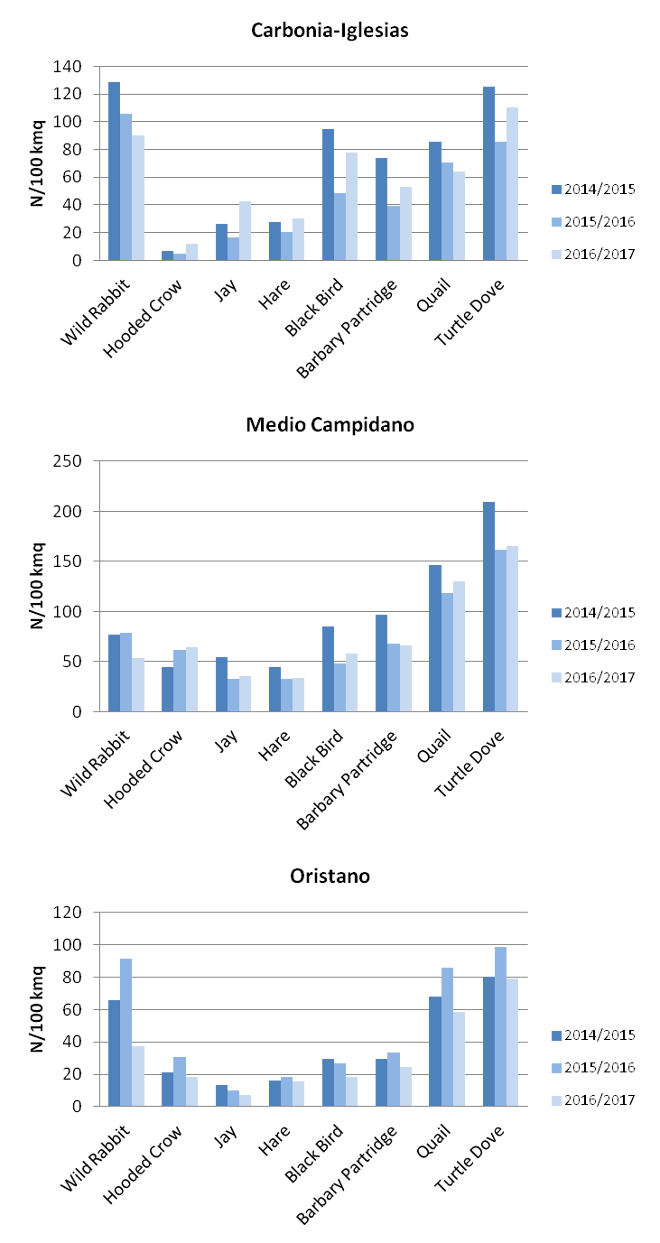
Fig.6 a. and b. Density of prey species according to hunting bags, provinces and hunting season.
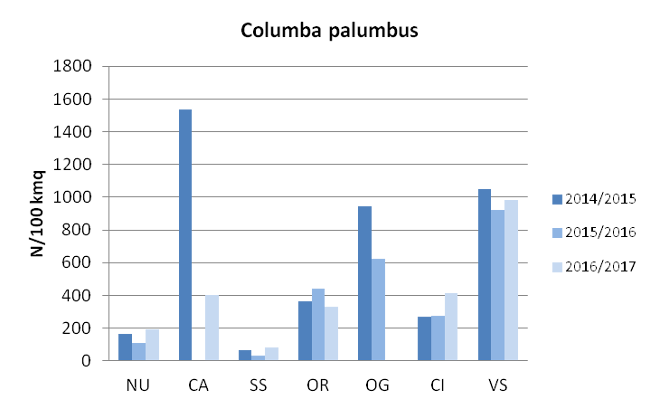
Density computed on hunting bags shows greater values for wood pigeon (Fig. 6) in all the provinces considered.
3.5 Golden eagle distribution
 Presence of reproductive golden eagle pair might represent a serious problem for the success of reintroduction, since it is likely to prevent the Bonelli’s eagle to establish a territory.
Presence of reproductive golden eagle pair might represent a serious problem for the success of reintroduction, since it is likely to prevent the Bonelli’s eagle to establish a territory.
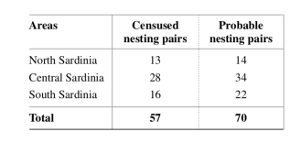
According to recent literature, Sardinia host a minimum of 57 breeding pairs (70 estimated), particularly concentrated in central area.
The presence of golden eagle nesting pairs has been a guiding principle for the choice of the release sites. At local level, unpublished data on golden eagle nesting site have also been collected and a minimum distance of 3 km from the release site has been considered as sufficient to avoid competition between them. It should also be considered that the two species have coexisted in the region for a long time and that, thanks to the orography of the region, an adequate availability of nesting site can be supposed and favour the coexistence of the two species.
3.6 Compliance with IUCN criteria for reintroduction
Reintroduction is the intentional movement and release of an organism inside its indigenous range from which it has disappeared. The following points have been defined by IUCN to decide if a reintroduction is an acceptable option; below they are reported as applicable to the reintroduction of Bonelli’s eagle in Sardinia. The criteria have been also discussed within the project itself: the project has been revised and approved by the LIFE programme of the EU which also requires a justification of fulfilling to IUCN conditions.
(1) the actions are justified, expected to yield quantitative conservation benefit (…) and have a high chance of success (…):
The reintroduction of the Bonelli's Eagle according to the proposed approach, have been carried out in the past in different areas in Spain within the framework of the LIFE project Bonelli, on the basis of the extensive experience of GREFA. The reintroduction in an insular area has been also realized, always within the scope of the same LIFE Project, in Majorca, with very positive results. These experiences help to ensure the availability of precise and detailed technique for the implementation of the species reintroduction.
(2) the translocated organisms are likely to be able to cope with new pathogen (…):
Currently in Sardinia it is not reported any pathogen that can jeopardise the success of reintroduction. The last discoveries of West Nile Disease date back to 2014 in the provinces of Nuoro and Oristano ( http://ecdc.europa.eu/en/healthtopics/west_nile_fever/West-Nile-fever-maps/PublishingImages/ECDC_WNF_Affected_current_and_past_seasons.png) The risk for the species in the event of new cases is however reduced because the infection is linked to the presence of vector insects (mosquitoes, mainly genus Culex); given the ecology of the species, direct infection by the mosquito to eagle can be considered highly unlikely while the possible secondary infection from capturing and feeding on infected corvids appears less unlikely. However, it should be pointed out that - until today - the viral strains isolated in Italy do not exhibit the same characteristics of lethality found in epidemics of North America and the Middle East.
Currently, there are no noticeable mortality rates in populations of sensitive birds (magpies and crows). To confirm this, health surveillance on wild species is based on active sampling (random sampling of apparently healthy animals and sentinel animals), due to the fact that episodes of mass lethality determined by the virus have never been found in the infected or at risk area of the country.
(3) alternatives to translocation have been assessed as less effective (…):
Bonelli’s eagle reintroduction in Sardinia is necessary to bridge the gap that is currently present in the distribution of the species and the need for the ecosystem to count with this super-predator. A natural recolonization would be extremely slow for the following reasons: - current Italian population, present only in Sicily, is itself numerically very reduced, due to factors exclusively depending upon human pressure. The availability of unoccupied territories in Sicily is itself very high and such as to be able to fully absorb the component of the population that enters into the new breeding cohorts. This makes it even lower the probability that subjects from Sicilian population disperse to Sardinia. Similar considerations can be made for the southern France population, and, a fortiori, for the far more abundant Spanish population, also considering the longer distances.
Observations of Bonelli’s Eagles in dispersion at a large distance from the reproductive areas are extremely rare in Italy, and even more if islands are considered (the sea poses an important barrier to their movements). Therefore, spontaneous re-colonization of Sardinia by the target species is highly improbable and didn’t occur, as far as can be known, since the species extinction in nineties. Population declines, non migratory and philopatric habits of the species contributes to make spontaneous recolonization improbable.
(4) the actions target areas where the causes of extinction of the species have been eliminated:
The extinction causes in Sardinia are linked mainly to the depletion of the population as a result of persecution and direct subtraction of chicks, for collection and falconry. Such practices however have been increasingly abandoned in the island also thanks to a cultural attitude radically changed towards wildlife, also thank to the generational replacement occurred in the last decades.
(5) the removal of individuals from their present habitat (…) is only considered if it does not endanger the wild source populations:
Bonelli’s eagles belonging to the following categories will be released in Sardinia:
- not yet fledged chicks, wild captured in Spain from nests characterized by high reproductive output (two chicks, the youngest of which generally is under the concrete risk of mortality before fledging), following formal authorization from the local competent Authority (Andalucia Environmental Ministry) and from the Spanish Environmental Ministry;
- captive bred chicks from GREFA and other captive breeding facilities (such as Christian Pacteau, in Vendée, France), both part of the AQUILA a LIFE project;
- chicks taken illegally from nests and subsequently seized by the competent authorities;
- individuals already able to fly recovered following seizures or rehabilitated in recovery centres;
As for genetic compliance, Mira et. al (2013) findings suggest that western Mediterranean Bonelli's Eagles may have a large-scale metapopulation structure, with subpopulations connected to some extent by distance-dependent dispersal, probably influenced by natal philopatry and the geographical configuration of subpopulations. Authors found a moderate genetic differentiation between subpopulations sampled in the western (Iberia and Morocco) and eastern (Cyprus) Mediterranean, whereas differentiation among subpopulations in the former region was weak to moderate and followed a pattern of isolation by distance. Only the small, peripheral and ecologically unique population of southwest Portugal had the lowest genetic diversity and the highest differentiation. Although Bonelli’s eagle dispersal may be behaviourally restricted because most individuals tend to breed close to their subpopulation of origin (i.e. exhibit natal philopatry; Greenwood & Harvey 1982), with females dispersing farther than males, individuals are capable of large-scale dispersal (Real & Mañosa 2001, Cadahía et al. 2010) and territorial recruitment sometimes occurred hundreds of kilometres from an individual's place of birth (Cheylan et al. 1996, Cadahía et al. 2009, Hernández-Matías et al. 2010). Hernández-Matías et al. (2013) reached similar conclusions: all local populations in western Europe belong to a single, spatially structured population operating as a source–sink system, whereby the populations in the south of the Iberian Peninsula act as sources and, thanks to dispersal, sustain all other local populations, which would otherwise decline. Sardinia is located in western Mediterranean and it’s highly probable that Bonelli’s eagle population belonged to the same metapopulation. In fact, large scale dispersal from Spain to Italy has been occasionally documented.
(6) the actions target areas (…) meet (…) the conditions necessary for the survival of a viable population of the species:
In the past, the species was widespread throughout the region. The shape and the abundance of mountainous massifs determine a high availability of suitable sites for nesting; the abundance of prey species does not appear to be a limiting factor for the vitality of the population in the long term. It must also be highlighted that local extinction is not linked to habitat deterioration but to direct persecution. In this regard, the presence of several protected areas, regional parks and/or oasis, coupled with the N2000 network should ensure a long term conservation of the species.
(7) a prior agreement between all parties involved (e.g. between the competent authority for the donor population and the manager of the area of reintroduction) has been concluded and documented:
Grefa and Christian Pacteau are project coordinator and associated beneficiary respectively of the project “Aquila a- LIFE”. Junta de Andalucia gave its support to provide chicks. At local level, Sardinia regional administration has given its formal support to the project and Fo.Re.S.T.A.S. will be involved through a formal agreement in the reintroduction activities. Authorization requests have been already sent to the Spanish competent administrations, quoted above.
(8) the actions target only areas where the attitude of the local population towards the planned reintroduction is favourable (…):
Several release sites have been already evaluated throughout Sardinia. The most suitable ones will be hopefully used progressively during the project, since it is necessary to change release site once a reproductive pair establishes its territory. Notably, the site chosen for the first release is Crastazza, located in Bitti municipality, Tepilora Regional Park (NU). Although several awareness raising and sensibilization activities are planned in the area, the presence of the regional park can be considered as a positive local attitude towards nature conservation. Previous experiences, like those gained under another project Life “Under Griffon wings”, show the efficacy of the sensibilization activities as well as the importance of the local attitude and have already created a positive background in another suitable release site (Bosa). Lastly, Bonelli's Eagle is known to prey actively on Corvids, habit that represents a positive value for the local community of hunters, owing to the many efforts that are regularly engaged to active control these species.
(9) (...) organisms are only reintroduced in areas where they previously occurred (..).
All the territory of the island of Sardinia is part of the historical distribution range of the Bonelli’s eagle.
(10) the proposal must include a preparatory phase, a re-introduction phase and a follow-up phase, as well as of an exit strategy, in case the translocation/reintroduction does not proceed according to plan.
According to the project, the preparatory phase is currently ongoing and foresees the editing of the feasibility study (present document) with activities plan, and the choice of the release sites. The reintroduction phase should start on May-June 2018 and last for the whole project duration. GPS data coming from released animals will strongly help the follow up phase and will be used to redirect the activities (notably, the reintroduction itself), if needed.
4 OPERATIONAL PLAN
4.1 Reintroduction technique
Reintroduction technique has been fine tuned by COFIB (Regional Government of the Balearic Islands) and GREFA and widely tested within the project Life “Bonelli”. The most successful technique foresees the release through closed hacking (Viada & Iglesias 2017) that means that chicks are kept since their arrival into a small closed nest located inside a bigger closed cage. This technique has been reported to be more successful than other approaches (e.g. open or elevated cage) ensuring the survival of chicks until their reach an adequate degree of independence. The cage is not fixed on the ground and can be removed, if needed (fig. 9).
 |
 |
| Fig.9. Hacking cage (on the left) and detail of the nest (on the right), inside the cage. | |
Hacking starts from the end of April to mid June, date largely depending upon availability of chicks. Chicks arrive at about 50 days of life, when they are capable of thermoregulation and feed, and are placed into nest. Approximately 7-10 days after, they are released into the main cage. About a month later, when they got hunting capabilities, fully developed the ability to fly and use perches, the cage can be opened so that the animal are free to fly outside. From this moment onwards, they will use the external platforms for food; during the first month after the release, they tend to remain within 1km from the cage, gradually moving further, making exploration flights. From mid-August to mid-September the dispersion phase begins (Viada & Iglesias 2017).
Animals are fed both during hacking and after the release, avoiding any visual contact whit human beings. Food will be also provided once the animals have established a territory, even if in a lesser amount than during the hacking phase. All released eagles will be provided with GPS-GSM transmitters in order to check their movements and, once established, to monitor pair formation and reproductive attempts.
4.2 Rules and responsibilities
GREFA as project coordinator, will be responsible to provide the animals to be released in Sardinia. Eagles will be ringed and tagged before their arrival in Sardinia, to avoid an additional manipulation upon arrival.
ISPRA will be in charge of
i) the activities related to animals transfer from Spain to Sardinia and release, including permits and authorizations;
ii) care of the animals during the hacking phase and after;
iii) monitoring of the released animals;
iv) education and public awareness campaigns in Sardinia.
Forestas, through a formal agreement with ISPRA, will be responsible of
i) the cage construction and maintenance. If needed, the cage might be moved to other suitable hacking site in subsequent years
ii) the purchase of the prey species to feed the animals
iii) veterinary treatment of the animals, if needed.
LITERATURE CITED
Apollonio M., Cossu A., Luccarini S., Carlini E., Chiarenzi B., 2010. Piano Faunistico Venatorio Regionale. Regione Sardegna, 268 pp.
Arroyo, B., Ferreiro, E., Garza, V., 1995. El Águila perdicera (Hieraaetus fasciatus) en España. Censo, reproducción y conservación. Serie Técnica. ICONA. Madrid.
Balbontín, J, Penteriani, V., Ferrer, M., 2003. Variations in the age of mates as an early warning signal of changes in population trends? The case of Bonelli’s eagle in Andalusia. Biol. Conserv., 109: 417-423.
Balbontín, J., Ferrer, M., 2009. Movements of juvenile Bonelli's eagles Aquila fasciata during dispersal. Bird Study, 56 (1): 86-95.
Bezzel E., 1957. Beiträge zur Kenntnis der Vogelwelt Sardiniens. Anz. Orn. Ges. Bayern , 4: 589-707.
BirdLife International, 2004. Birds in Europe: population estimates, trends and conservation status. Cambridge, UK. BirdLife International.
BirdLife International, 2017. European birds of conservation concern: populations, trends and national responsibilities. Cambridge, UK: BirdLife International.
BirdLife International, 2018. IUCN Red List for birds. Downloaded from http://www.birdlife.org on 15/03/2018.
Cadahía, L., López-López, P., Urios, V., Soutullo, A. & Negro, J.J. 2009. Natal dispersal and recruitment of two Bonelli's Eagles Aquila fasciata: a four-year satellite tracking study. Acta Ornithol. 44: 193-198.
Cadahía, L., López-López, P., Urios, V. & Negro, J.J. 2010. Satellite telemetry reveals individual variation in juvenile Bonelli’s eagle dispersal areas. Eur. J. Wildlife Res. 56: 923-930.
Camarda I., Laureti L., Angelini P., Capogrossi R., Carta L., Brunu A., 2015. “Il Sistema Carta della Natura della Sardegna” ISPRA, Serie Rapporti, 222/2015.
Cara G., 1842. Elenco degli uccelli della Sardegna, Torino.
Carrascal, L. M., Seoane, J., 2009. Factors affecting large-scale distribution of the Bonelli's eagle Aquila fasciata in Spain. Ecological Research, 24 (3): 565-573.
Carrete M., Sánchez-Zapata J.A., Tella J.L., Gil- Sánchez J.M., Moleon M., 2006. Components of breeding performance in two competing species: habitat eterogeneity, individual quality and density-dependence. Oikos 112: 680-690.
Cheylan, G., Ravayrol, A., Cugnasse, J.-M., Billet, J.-M. & Joulot, C. 1996. Dispersion des Aigles de Bonelli Hieraaetus fasciatus juvéniles bagués en France. Alauda 64: 413-419.
Cramp, S. and Simmons, K.E.L., 1980. The Birds of the Western Palearctic. Vol. II, Oxford University Press, Oxford.
Ferguson-Lees J., Christie D. A., 2001. Raptor of the World. Houghton Mifflin Harcourt, 992 pages.
Fernández, A., De la Torre , J. A.., Ansola, L. M., 1993. El Águila de Bonelli (Hieraaetus fasciatus) en Burgos. Situación, reproducción, alimentación suplementaria y problemática. Consejería de Medio Ambiente, Junta de Castilla y León, Valladolid.
Greenwood, P.J. & Harvey, P.H. 1982. The natal and breeding dispersal of birds. Ann. Rev. Ecol. Evol. Syst. 13: 1-21.
Hernandez A., Real J., Pradel R., 2011. Analyse démographique des populations d’Agle de Bonelli de France, Catalogne et d’autres régions de la péninsule ibérique: analyse de la survie et viabilité des populations. In: Scher O. & Lecacheur (eds.) 2011. La conservation de l’Aigle de Bonelli. Actes du colloque International, 28 et 29 janvier 2010, Montpellier. CEN LR, CEEP, CORA FS & DREAL LR. Pp: 23-26.
Hernández-Matías, A., Real, J., Pradel, R., Ravayrol, A., Vincent-Martin, N., Bosca, F. & Cheylan, G. 2010. Determinants of territorial recruitment in Bonelli’s eagle (Aquila fasciata) populations. Auk 127: 173-184.
Hernández-Matías, A., Real, J., Moleón, M., Palma, L., Sánchez-Zapata, J.A., Pradel, R., Carrete, M., Gil-Sánchez, J.M., Beja, P., Balbontín, J., Vicent-Martin, N., Ravayrol, A., Benítez, J.R., Arroyo, B., Fernández, C., Ferreiro, E., García, J., 2013. From local monitoring to a broad-scale viability assessment: a case study for the Bonelli's eagle in western
Europe. Ecol. Monogr. 83, 239–261.
Iezekiel S., Bakaloudis D.E., Vlachos C. G., 2011. Le regime alimentaire de l’Aigle de Bonelli Aquila fasciata sur l’ile de Chypre. In: Scher O. & Lecacheur (eds.) 2011. La conservation de l’Aigle de Bonelli. Actes du colloque International, 28 et 29 janvier 2010, Montpellier. CEN LR, CEEP, CORA FS & DREAL LR. Pp: 62-65.
IUCN/SSC, 2013. Guidelines for Reintroductions and Other Conservation Translocations. Version 1.0. Gland, Switzerland: IUCN Species Survival Commission, viiii + 57 pp
Kastritis T., 2011. Distribution et exigences écologique de l’Aigle de Bonelli Aquila fasciata dans le sud du Portugal. In: Scher O. & Lecacheur (eds.) 2011. La conservation de l’Aigle de Bonelli. Actes du colloque International, 28 et 29 janvier 2010, Montpellier. CEN LR, CEEP, CORA FS & DREAL LR. Pp: 31-33.
Kunkel P., 1963. Beitrag zur Avifauna Sardiniens. Vogelvelt 84: 137-145.
López-López P., 2011. Démographie et compétition entre l’Aigle de Bonelli Aquila fasciata et l’Aigle royal Aquila chrysaetos: implications pour la gestione t la conservation d’especès menacées. In: Scher O. & Lecacheur (eds.) 2011. La conservation de l’Aigle de Bonelli. Actes du colloque International, 28 et 29 janvier 2010, Montpellier. CEN LR, CEEP, CORA FS & DREAL LR. Pp: 53-57.
López-López, P., García-Ripollés, C., Urios, V., 2007. Population size, breeding performance and territory quality of Bonelli's Eagle Hieraaetusfasciatus in eastern Spain . Bird Study, 54 (3): 335-342.
Massa B 1976. Una specie in via di estinzione: l’Aquila del Bonelli. In: Pedrotti F. (red.), S.O.S. fauna. Animali in pericolo in Italia. W.W.F. Italia, Camerino.
Mira S., Arnaud-Haond S., Palma L., Cancela M.L., Beja P., 2013. Large-scale population genetic structure in Bonelli's Eagle Aquila fasciata. Ibis 155(3):485–498.
Mocci Demartis A. & Restivo De Miranda M.A., 1978. Contributo allo studio dei mallofagi di rapaci diurni. Gli Uccelli d'talia III (4): 160-167.
Mocci Demartis A., 1980. Nuove segnalazioni dalla Sardegna di specie ornitiche accidentali, o migratrici irregolari, o nidificanti, comunque in diminuzione. Riv. Ital. Ornit. 50 (4): 203-220.
Moleón, M., Gil-Sánchez, J. M., Real, J., Sánchez-Zapata, J. A., Bautista, J., Sánchez-Clemot, J. F., 2007. Ecología trófica de las águilas-azor perdiceras Hieraaetus fasciatus territoriales durante el periodo no reproductor en la Península Ibérica. Ardeola, 54 (1): 135-143.
Moltoni E. & Sciacchitano J., 1926. Atti Soc. Ital. Sci. Nat., 55: 154-184.
Moltoni E., 1971. Gli uccelli ad oggi riscontrati nelle isole di Tavolara, Molara e Molarotto (Sardegna nord - orientale) Riv. Ital. Ornit. 41 (2): 223-372.
Nardelli R., Raganella E., Serra L., Spina F., 2018. The reintroduction of Bonelli’s Eagle (Aquila fasciata Vieillot 1822) in Sardinia: hacking site selection. Action A2, LIFE PROJECT “AQUILA a-LIFE”, March 2018
Ontiveros D., 2016. Águila perdicera – Hieraaetus fasciatus. En: Enciclopedia Virtual de los Vertebrados Españoles. Salvador, A., Morales, M. B. (Eds.). Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org
Ontiveros D., Caro, J., Pleguezuelos, J. M., 2005. Prey density, prey detectability and food habits: the case of Bonelli’s agle and the conservation measures. Biol. Conserv., 123: 19-25.
Ontiveros, D., Pleguezuelos, J. M., 2003. Influence of climate on Bonelli’s eagle (Hieraaetus fasciatus V.) breeding success trough the Western Mediterranean . J. Biogeog., 30 (5): 755-760.
Palma L., Dias A., Cangarato R., Pais M., 2011. Spécificités et dynamique de la population d’Aigle de Bonellli Aquila fasciata dans le Sud de Portugal. In: Scher O. & Lecacheur (eds.) 2011. La conservation de l’Aigle de Bonelli. Actes du colloque International, 28 et 29 janvier 2010, Montpellier. CEN LR, CEEP, CORA FS & DREAL LR. Pp: 28-30.
Parellada X., De Juan, A., Alamany O., 1984. Ecologia de l´aliga cuabarrada (Hieraaetus fasciatus): factors limitants, adaptacions morfológiques i ecológiques i relacions interespecífiques amb l´aliga daurada (Aquila chrysaetos). Rapinyaires Mediterranis, 2: 121-141.
Ponchon C., 2011. Répartition mondiale et evolution des populations méditerranéennes d’Aigle de Bonelli Aquila fasciata. In: Scher O. & Lecacheur (eds.) 2011. La conservation de l’Aigle de Bonelli. Actes du colloque International, 28 et 29 janvier 2010, Montpellier. CEN LR, CEEP, CORA FS & DREAL LR. Pp: 14-15.
Ravayrol A., 2011. Quelles conditions poour maintien et la reconquête de territoire par l’espèce?. In: Scher O. & Lecacheur (eds.) 2011. La conservation de l’Aigle de Bonelli. Actes du colloque International, 28 et 29 Janvier 2010, Montpellier. CEN LR, CEEP, CORA FS & DREAL LR. Pp: 133-136.
Real J. 2003. Águila-Azor Perdicera. Hieraaetus fasciatus. En: Martí, R., Del Moral, J. C. (Eds.). Atlas de las aves reproductoras de España. Dirección General de Conservación de la naturaleza-Sociedad Española de Ornitología, Madrid. Pp. 192-193.
Real, J., Mañosa, S., Codina, J. 1998. Post-nestling dependence period in the Bonelli’s eagle Hieraaetus fasciatus. Ornis fennica, 75: 129-137.
Real, J. & Mañosa, S., 2001. Dispersal of juvenile and immature Bonelli's Eagles in northeastern Spain. J. Raptor Res. 35: 9-14.
Resano J., 2011. L’utilisation des isotopes stables (δ13C, δ15N, δ34S) dans l’étude du regime alimentaire de l’Aigle de Bonelli Aquila fasciata in Catalogne. In: Scher O. & Lecacheur (eds.) 2011. La conservation de l’Aigle de Bonelli. Actes du colloque International, 28 et 29 janvier 2010, Montpellier. CEN LR, CEEP, CORA FS & DREAL LR. Pp: 58-61.
Rondinini, C., Battistoni, A., Peronace, V., Teofili, C. (compilatori). 2013. Lista Rossa IUCN dei Vertebrati Italiani. Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Roma.
Rosario I., Cardoso P., Tomé R., Palma L., 2011. Comportament territorial d’une populations arboricole d’Aigle del Bonelli Aquila fasciata dans le sud-ouest du Portugal. In: Scher O. & Lecacheur (eds.) 2011. La conservation de l’Aigle de Bonelli. Actes du colloque International, 28 et 29 janvier 2010, Montpellier. CEN LR, CEEP, CORA FS & DREAL LR. Pp: 46-52.
Ruiu D. 2017. Status of Golden Eagle Aquila chrysaetos nesting pairs in Sardinia. Avocetta 41: 89-91.
Schenk H. 1972. Coloro che cavalcano il vento. Situazione faunistica dei rapaci in Sardegna e proposte per la loro salvaguardia. Pro Avibus, boll. Della Lega Naz. contro distr. Uccelli n.3/4: 4-8.
Schenk H., 1976. Analisi della situazione faunistica in Sardegna. Uccelli e Mammiferi. Pp. 502-503. In: S.O.S. Fauna. Animali in pericolo in Italia. WWF-Italia, Camerino.
Shirihai, H., R. Yosef, D. Alon, G. M. Kirwan, and R. Spaar. 2000. Raptor migration in Israel and the Middle East: a summary of 30 years of field research. International Birding and Research Center, Eilat, Israel.
Steinbacher, 1952. Zur Verbreitumg und Biologie der Vögel Sardiniens. Vogelwlt 63: 197-208.
Sudhaus W., 1966.Ornithologische Beobachtungen im April auf Sardinien. Ornitologische Mitteilungen 18 (5):87-100.
Torre A., 1980. Osservazioni sulla avifauna della Nurra. Boll. Soc. Sarda Sci. Nat., 19: 141-170.
Toso S., 1972. Osservazioni di rapaci diurni in Sardegna. Riv. Ital. Ornit. 42 (2): 435-444
Tucker G. M., Heath M. F., 1994. Bird in Europe: their conservation status. Cambridge, UK. BirdLife International.
Viada C., Iglesias J.J., 2017. La jaula hacking: nuevo sistema para el LIFE BONELLI. Unpublished report, Life Bonelli.
